Fastep COVID-19 Antigen Home Test

How to Use
Document Download
FAQs
Contact Us
How to order
How to Use
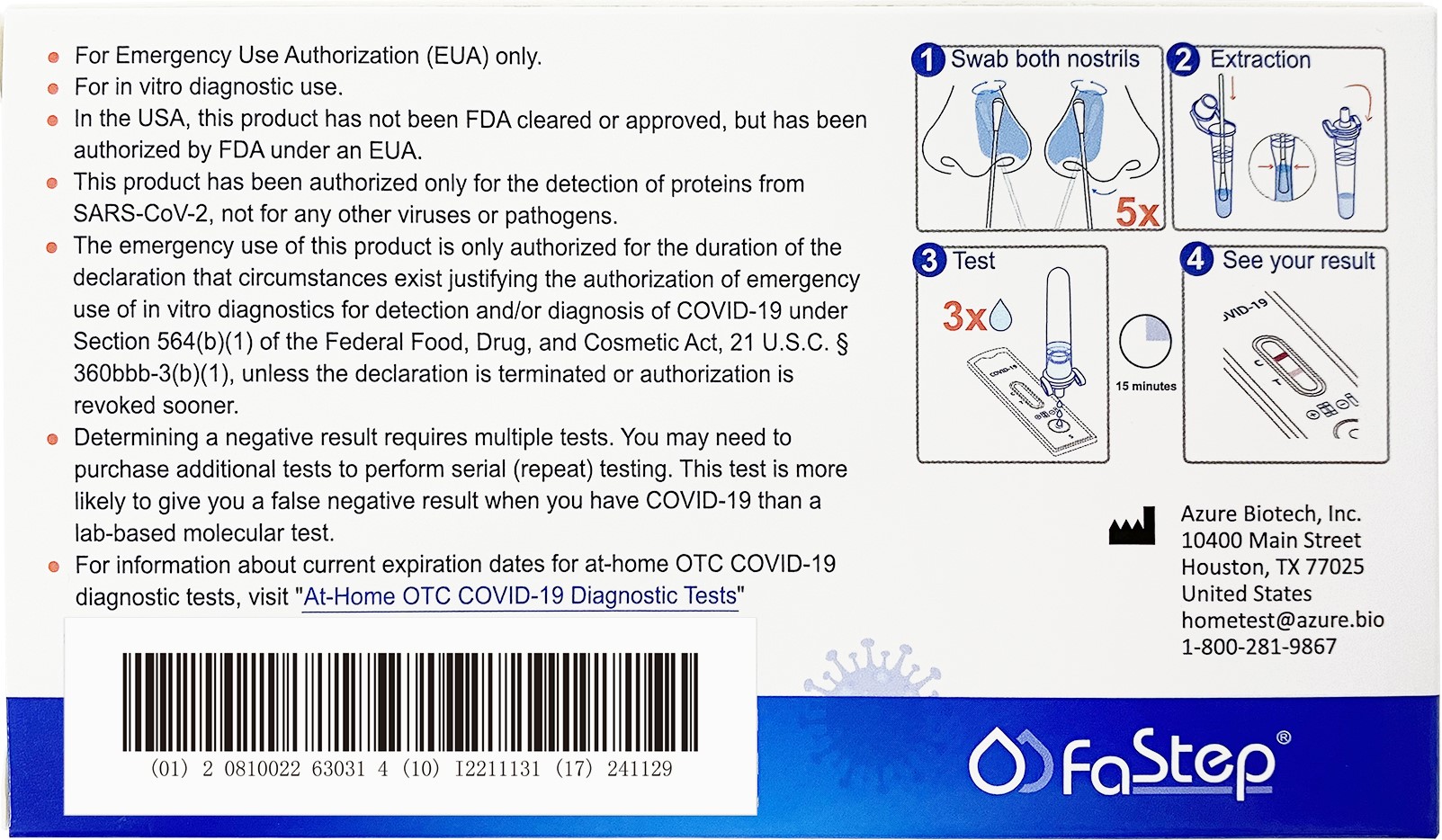
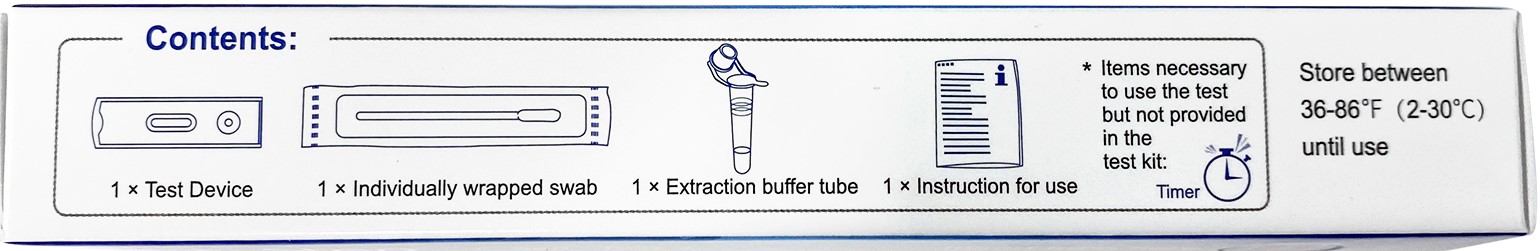



Document Download
EUA letter
Fastep COVID-19 Antigen Home Test Instructions for Use
Fastep COVID-19 Antigen Home Test Instructions for Use for Healthcare Professionals
Fastep COVID-19 Antigen Home Test Fact Sheet for Healthcare Professionals
Fastep COVID-19 Antigen Home Test Instructions for Use – Spanish Version
FAQs
For more information on EUAs go here: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
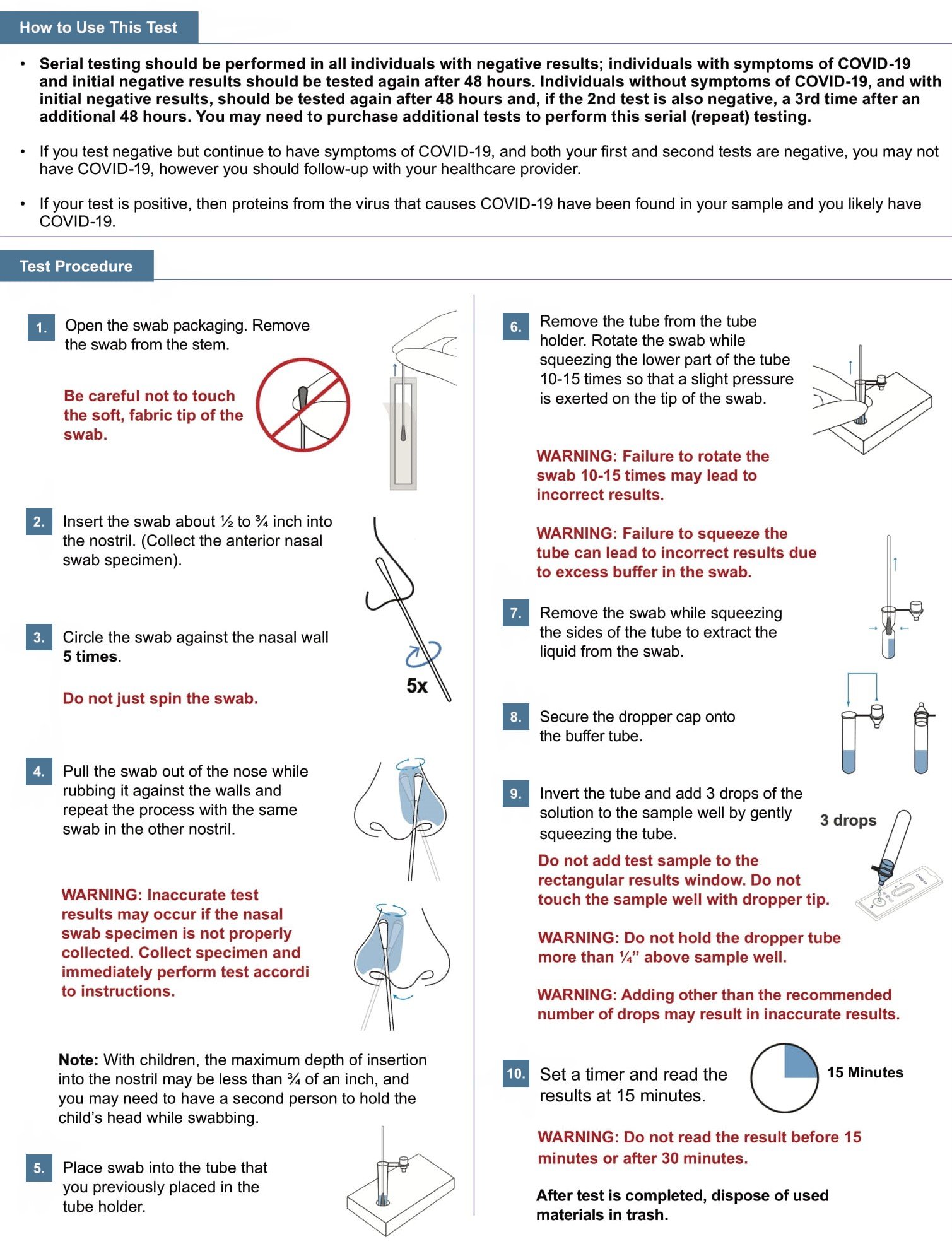
There are different kinds of tests for the SARS-CoV-2 virus that causes COVID-19. Molecular tests detect genetic material from the virus. Antigen tests, such as the Fastep COVID-19 Antigen Home Test, detect proteins from the virus. Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
Clinical studies have shown that antigen tests more accurately determine whether you are infected with the virus that causes COVID-19 when taken multiple times across several days. Repeat testing improves test accuracy. This serial testing approach is recommended to minimize the risk of incorrect results. For more information on the performance of the test and how the performance may apply to you, please refer to the performance data in the Healthcare Provider Instructions for Use (IFU), available at fastep.azure.bio.
A positive result means that it is very likely you have COVID-19 because proteins from the virus that causes COVID-19 were found in your sample. You should self-isolate from others and contact a healthcare provider for medical advice about your positive result.
A negative test result indicates that antigens from the virus that causes COVID-19 were not detected in your sample. However, if you have symptoms of COVID-19, and your first test is negative, you should test again in 48 hours since antigen tests are not as sensitive as molecular tests. If you do not have symptoms and received a negative result, you should test at least two more times with 48 hours in between tests for a total of three tests. If you have a negative result, it does not rule out SARS-CoV-2 infection; you may still be infected and you may still infect others. It is important that you work with your healthcare provider to help you understand the next steps you should take.
An invalid result means the test was not able to tell if you have COVID-19 or not. If the test is invalid, a new swab should be used to collect a new nasal specimen and you should test again with a new test.IMPORTANT: Do not use this test as the only guide to manage your illness. Consult your healthcare provider if your symptoms persist or become more severe. Individuals should provide all results obtained with this product to their healthcare provider.
Please Click here to report your test results.
Contact Us
hometest@azure.bio
Phone number
1-800-281-9867
Hours of operation
9 AM- 5 PM CST, Monday – Friday
How to order
List of distributors
This product has not been FDA cleared or approved, but has been authorized by FDA
under an EUA;
This product has been authorized only for the detection of proteins from SARSCoV-2, not for any other viruses or pathogens; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
This product has been authorized only for the detection of proteins from SARSCoV-2, not for any other viruses or pathogens; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
FAQs
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

